Do Oxygen and Strontium Form an Ionic Compound
A b c 3865 Å α β γ 60 cell volume 4083 Å 3 cell occupancy is shown in Table 234. Does Oxygen and iodine form a ionic compound.
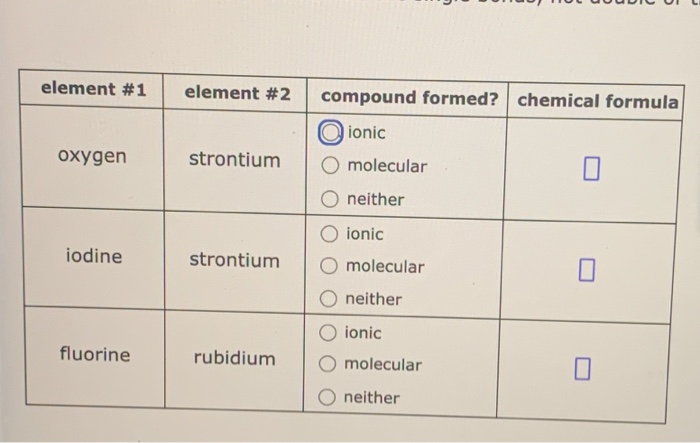
Solved Element 1 Element 2 Compound Formed Chemical Chegg Com
Except where otherwise noted data are given for materials in their standard state at 25 C 77 F 100 kPa.
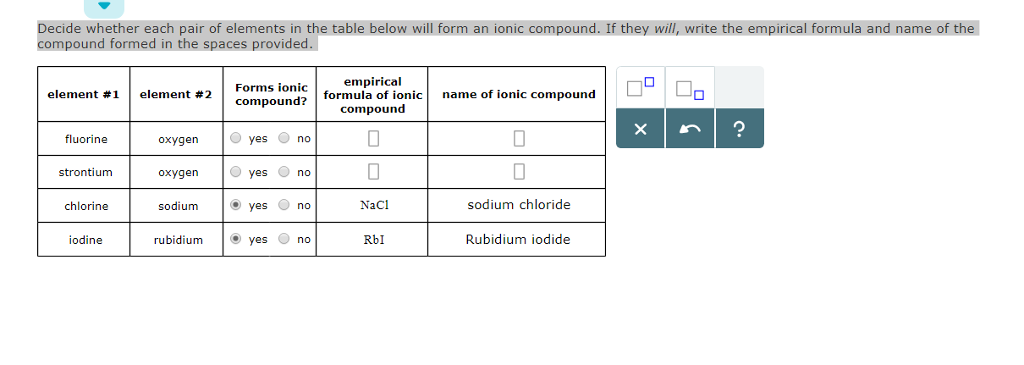
. Want to see the full answer. It has two valence electrons and commonly forms Sr2 ion. TYPES OF COMPOUNDS Ionic compounds are compounds composed of ions charged particles that form when an atom or group of atoms gains or loses electrons.
The compound is ionic in nature because it contains a metal aluminum and a non-metal oxygen. The I ionic compound is. Rubidium oxide is an example of an ionic bond between Rubidium and Oxygen demonstrated by the molecular formula of Rb2O.
When strontium reacts with iodine to form an ionic compound. What is the for an ionic compound made of strontium and bromine. In general the chemistry of strontium is quite similar to that of calcium.
Li 2 Cr 2 O 7. What is strontium ion. Bromine is a non-metal with seven valence electrons and commonly forms the Br- ion.
Srs Br_2l rarr SrBr_2s. The Chemical Formula is SrO. The subscript for both calcium and oxygen is 1.
In its compounds strontium has an exclusive oxidation state of 2 as the Sr 2 ion. Li 2 O 2. CThe compound formed between rubidium and bromine is an ionic compound because rubidium is a metal and bromine is a non-metal.
Strontium iodide SrI2 is a salt of strontium and iodine. The cost on each the cation and anion is similar and is thus balanced. What is the ionic formula for strontium and oxygen.
Likewise does bromine and rubidium kind an ionic compound. 56 -63 and the 1411 Lab Manual p. Then identify the anion and write down its symbol and charge.
The subscript for each calcium and oxygen is 1. Want to see the full answer. The metal is an active reducing agent and readily reacts with halogens oxygen and.
I am taking Chem. Chemistry questions and answers. 1 Fluorine and Strontium forms an ionic compound Strontium Fluori View the full answer Transcribed image text.
The charge on both the cation and anion is same and is thus balanced. When strontium reacts with iodine to form an ionic compound. Lithium and oxygen 10.
Check out a sample QA here. Determine the chemical formula for the ionic compound that forms between cesium and bromine. Strontium Oxide is a compound of Oxygen andStrontium together.
It has a role as a cofactor. Element 1 element 2 Forms ionic compound. The metal always donates electrons to the non-metal to form the ionic bond.
Does oxygen and rubidium form an ionic compound. Each metal atom loses electron s each nonmetal atom games electron s There must be strontium atom s form every iodine atom s for every iodine atom s in the reaction. Well strontium is an alkaline earth from Group 2 of the Periodic Table.
Therefore it is most likely an ionic compound. CThe compound shaped between rubidium and bromine is an ionic compound as a result of rubidium is a steel and bromine is a non-metal. Li 3 PO 4.
When strontium and bromine make music together we conceive of a redox process. Sodium and nitrogen 9. Likewise does bromine and rubidium form an ionic compound.
Li 2 HPO 4. Determine the chemical formula for the ionic compound that forms between strontium and nitrogen. Strontium and oxygen can form an ionic compound with the name strontium oxide and formula SrO.
Empirical formula of ionic compound name of ionic compound fluorine strontium yes no chlorine rubidium yes no bromine oxygen yes no potassium fluorine yes no. Strontium2 is a strontium cation a divalent metal cation and a monoatomic dication. The oxidation number for oxygen in calcium-.
Li 2 CrO 4. FORMULAS AND NOMENCLATURE OF IONIC AND COVALENT COMPOUNDS Adapted from McMurryFay section 210 p. It is an ionic water-soluble and deliquescent compound that can be used in medicine as a substitute for potassium iodide.
In the case of aluminum it always forms an ion with a 3 charge when it forms an ion and oxygen always forms an ion with a -2 charge. Does Oxygen and iodine form a ionic compound. Students whove seen this question also like.
Check out a sample QA here. In its compounds strontium has an exclusive oxidation state of 2 as the Sr2 ion. The metal is an active reducing agent and readily reacts with halogens oxygen and sulfur to yield halides oxide and sulfide.
A stable bond between two elements has been formed and is resembled in a solid powder. CsCl Determine the chemical formula for the ionic compound that forms between sodium and oxygen. Explain how an ionic compound forms from these elements.
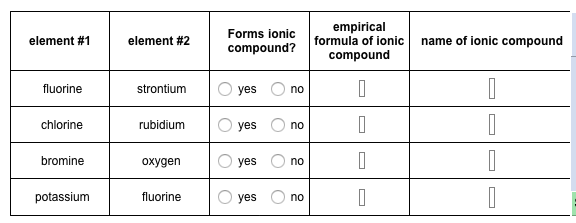
Solved Element 1 Element 2 Forms Ionic Compound Empirical Chegg Com
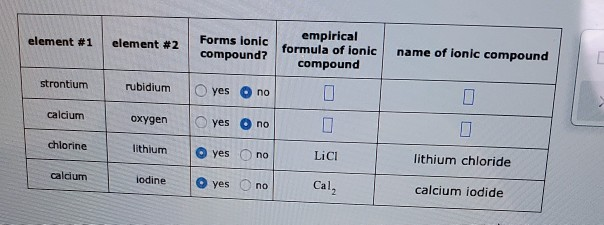
Solved Element 1 Element 2 Forms Ionic Compound Empirical Chegg Com
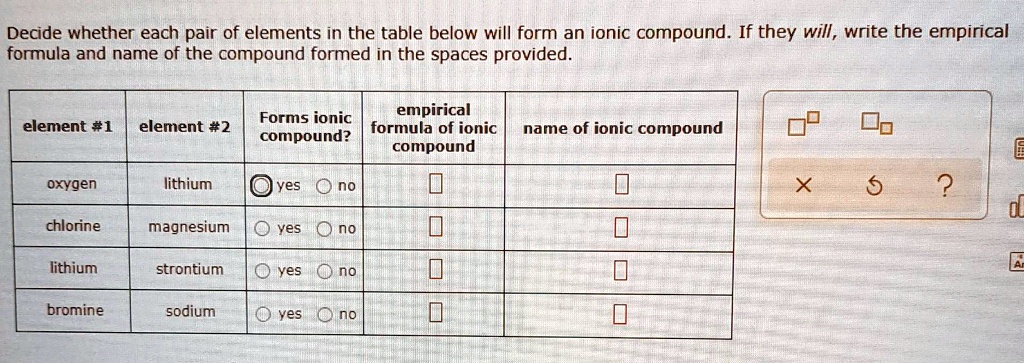
Solved Decide Whether Each Pair Of Elements In The Table Below Will Form An Ionic Compound If They Will Write The Empirical Formula And Name Of The Compound Formed In The Spaces Provided
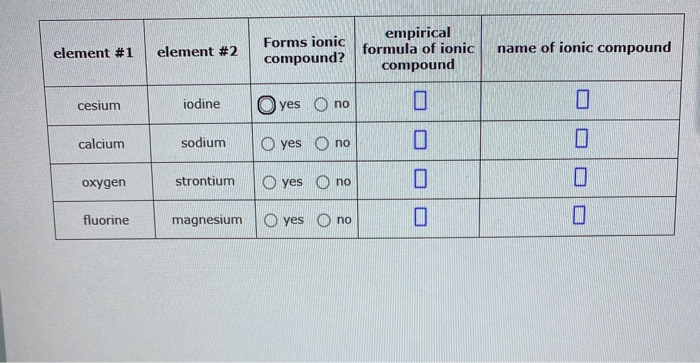
Solved Element 1 Element 2 Forms Ionic Compound Empirical Chegg Com
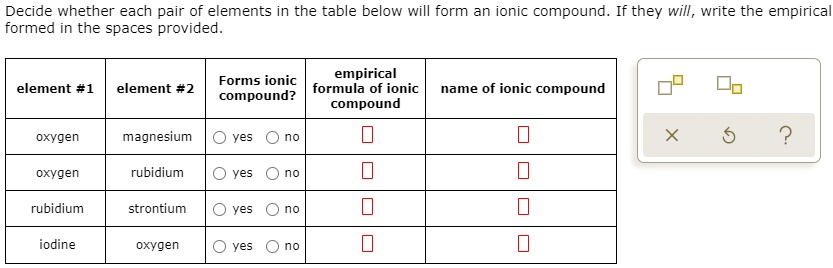
Solved Decide Whether Each Pair Of Elements In The Table Below Will Form An Ionic Compound If They Will Write The Empirica Formed In The Spaces Provided Forms Ionic Empirical Formula Of Ionic
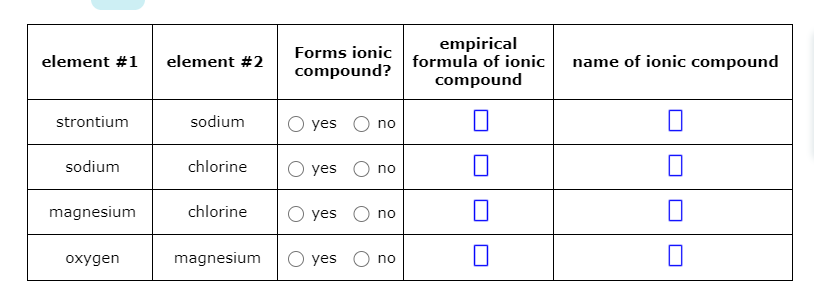
Solved Element 1 Element 2 Forms Ionic Compound Empirical Chegg Com
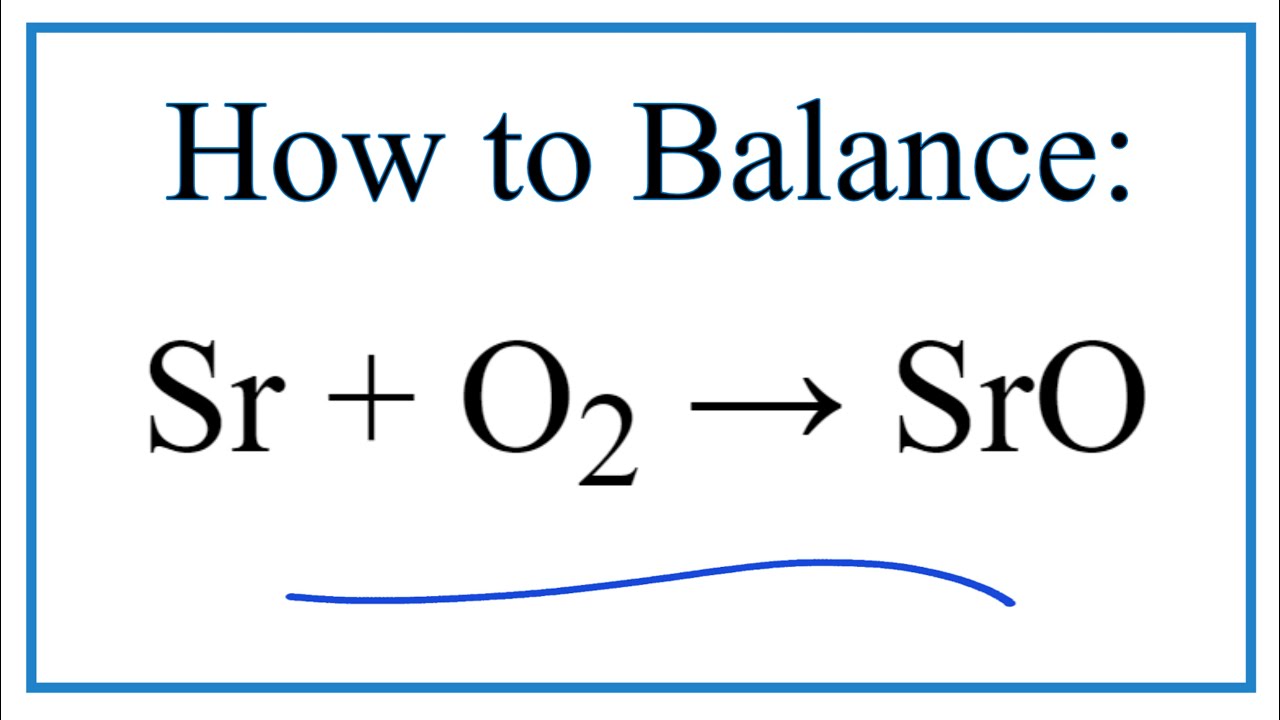
How To Balance Sr O2 Sro Strontium Metal Oxygen Gas Youtube
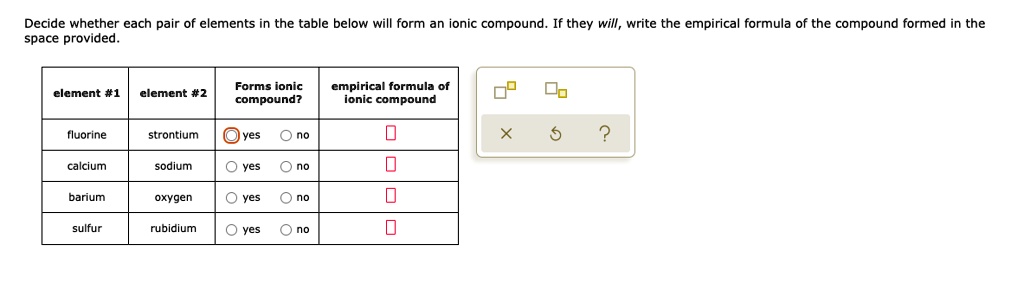
Solved Decide Whether Each Pair Of Elements In The Table Below Will Form An Ionic Compound Space Provided They Will Write The Empirical Formula Of The Compound Formed In The Forms Ionic
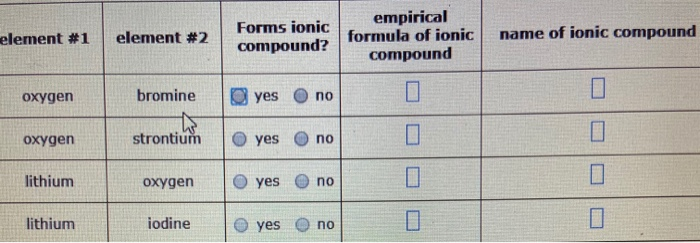
Solved Element 1 Element 2 Forms Ionic Compound Empirical Chegg Com
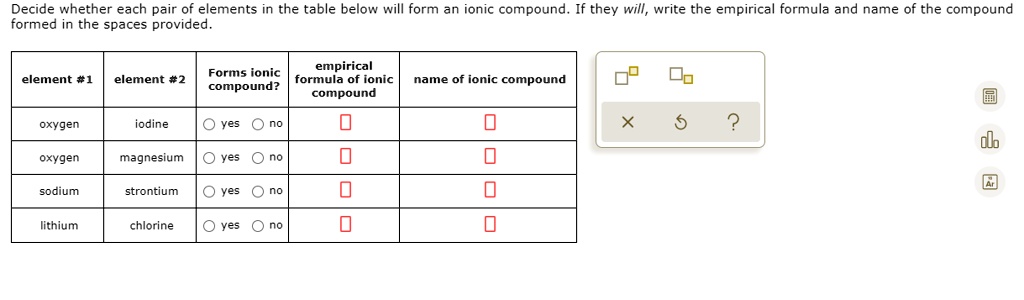
Solved Decide Whether Each Pair Ot Elements Formed In The Spaces Provided The Table Below Will Form An Ionic Compound If They Will Write The Empirical Formula And Name Of The Compound Empirical
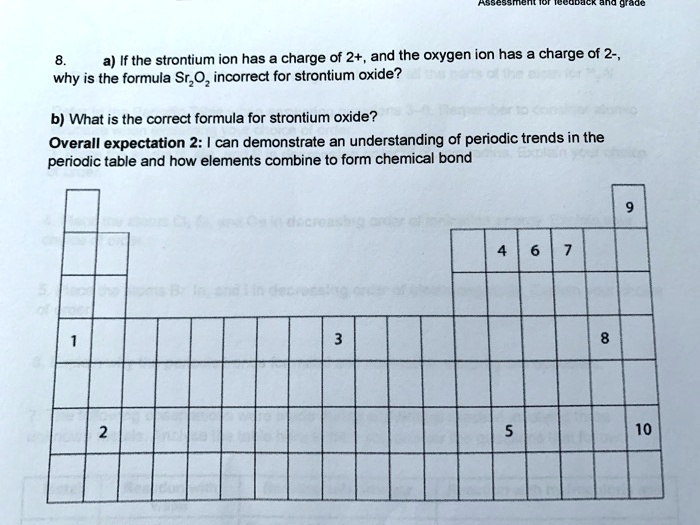
Solved A If The Strontium Ion Has Charge Of 2 And The Oxygen Ion Has Charge Of 2 Why Is The Formula Sr Oz Incorrect For Strontium Oxide B What Is The Correct Formula For
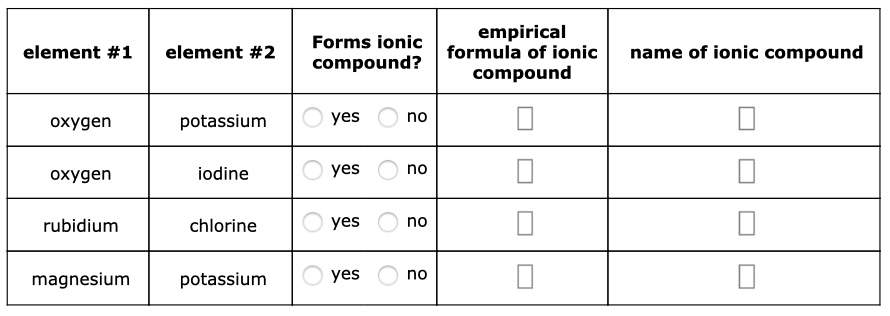
Answered Empirical Formula Of Ionic Compound Bartleby

Decide Which Element Probably Forms A Compound With Oxygen That Has A Chemical Formula Most And Homeworklib
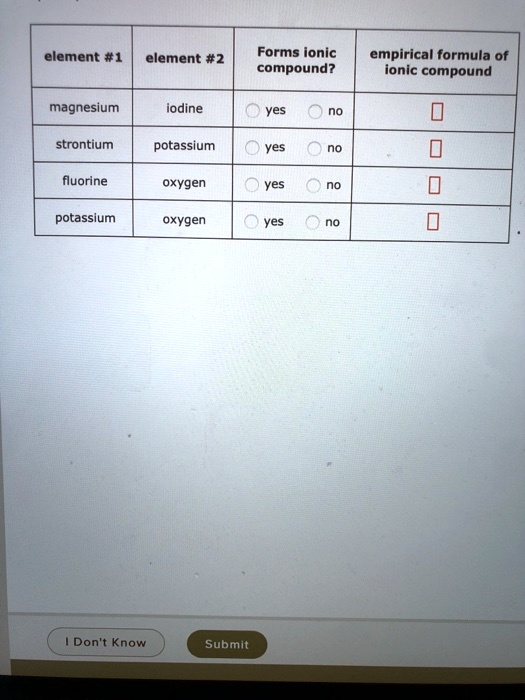
Solved Element 1 Element 2 Forms Ionic Compound Empirical Formula Of Ionic Compound Magnesium Iodine Yes Strontium Potassium Yes Fluorine Oxygen Yes Potassium Oxygen Yes Don T Know Submit
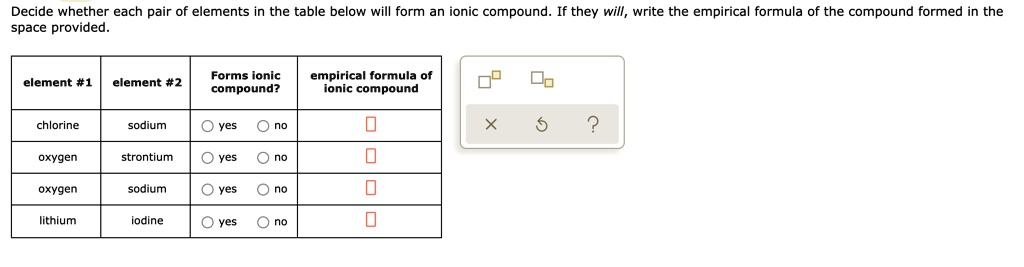
Solved Decide Whether Each Pair Of Elements In The Table Below Will Form An Ionic Compound They Will Write The Empirical Formula Of The Compound Formed In The Space Provided Forms Ionic Compound

Solved Decide Whether Each Pair Of Elements In The Table Chegg Com
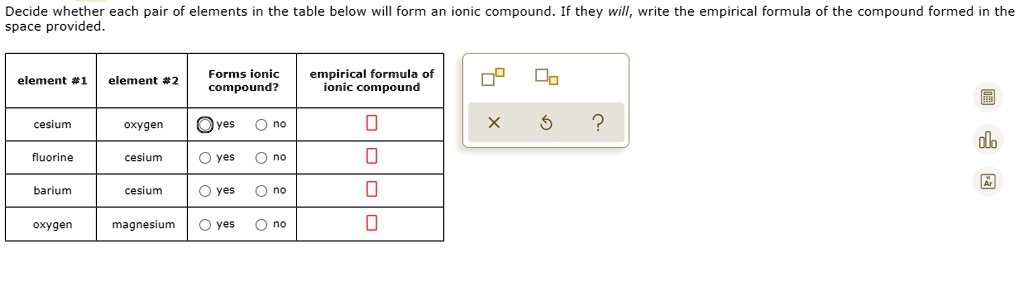
Solved Decide Whether Each Pair Of Elements In The Table Below Will Form An Ionic Compound If They Will Write The Empirical Formula Of The Compound Formed In The Space Provided Forms Ionic
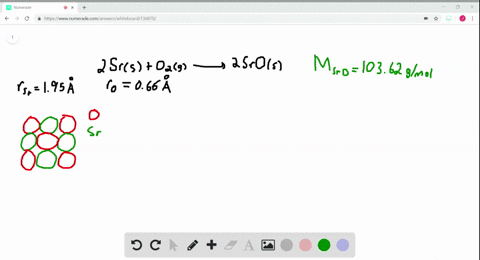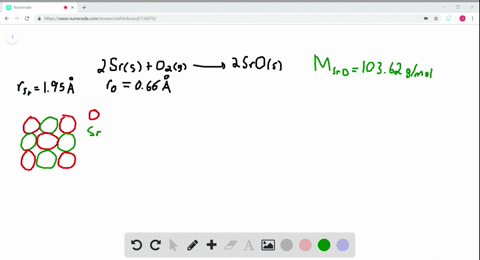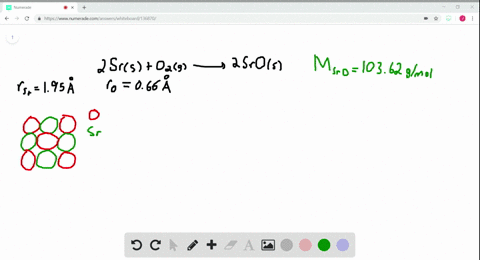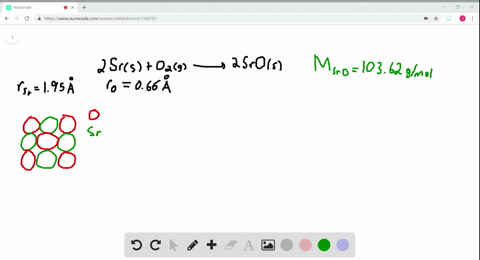
Solved The Ionic Substance Strontium Oxide Sro Forms From The Reaction Of Strontium Metal With Molecular Oxygen The Arrangement Of The Ions In Solid Sro Is Analogous To That In Solid Nacl A

Solved Decide Whether Each Pair Of Elements In The Table Chegg Com
Comments
Post a Comment